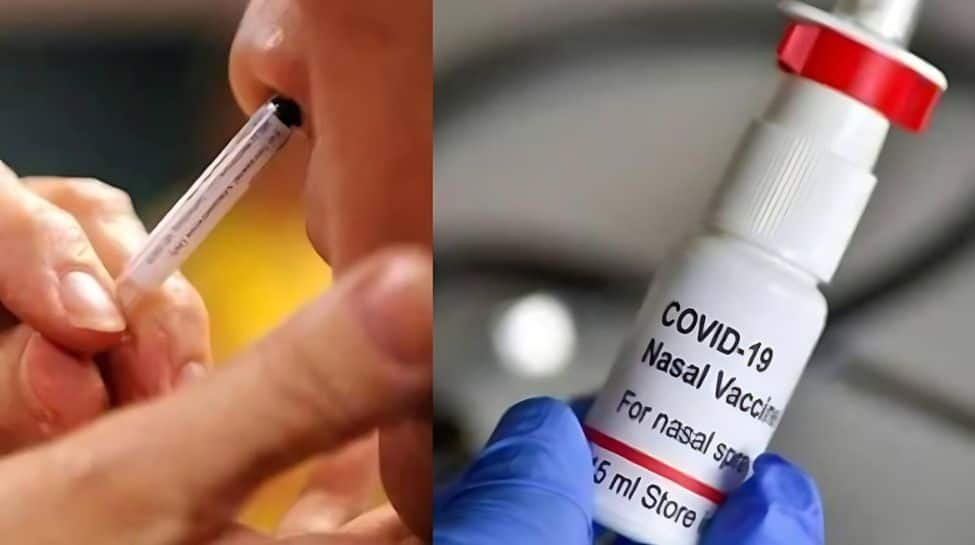
A novel, needle-free, nasally administered Covid-19 vaccine licensed by Hyderabad-based Indian Immunologicals is set to be a game-changer against the infectious disease, according to research Tuesday.
The study led by a team at Griffith University in Australia investigated the efficacy of administering a Covid vaccine – CDO-7N-1 – through the nostrils.
“This is a live attenuated intranasal vaccine, called CDO-7N-1, designed to be administered intranasally, thereby inducing potential mucosal immunity as well as systemic immunity with a single dose,” said Professor Suresh Mahalingam from Griffith’s Institute of Biomedicine and Glycomics.
The research, published in the journal Nature Communications, showed that the vaccine induces strong memory responses in the nasal mucosa, offering long-term protection for a year or more.
“It is designed to be administered in a single dose, ideally as a booster shot, as a safe alternative to needles and without short- or long-term adverse reactions,” Mahalingam said.
Compared with alternative vaccination strategies, live attenuated vaccines offer several important advantages.
Its effects on humoral and cellular immunity are strong and persistent, but often require a dose.
Unlike many other vaccine platforms that use a single antigen, live attenuated vaccines include the entire virus, resulting in broad immunity.
Dr. Xiang Liu, lead author of the study, noted that CDO-7N-1 “offers cross-protection against all variants of concern.” It also has neutralizing capacity against SARS-CoV-1, the respiratory disease responsible for the 2002-2004 SARS outbreak.
“The vaccine offers potent protection against transmission, prevents reinfection and spread of the virus, while reducing the generation of new variants,” Liu said.
“Unlike the mRNA vaccine, which targets only the spike protein, CDO-7N-1 induces immunity to all major SARS-CoV-2 proteins and is highly effective against all major variants to date. Importantly, the vaccine remains stable at 4 degrees Celsius for seven months, making it ideal for low- and middle-income countries,” Liu added.
The vaccine has been licensed to Indian Immunologicals, a major producer of vaccines for human and animal use.
Dr. K. Anand Kumar, CEO of Indian Immunologicals, said the company has “completed all necessary studies of the vaccine” and is now planning to launch “clinical trials.”
Disclaimer:
The information contained in this post is for general information purposes only. We make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the post for any purpose.
We respect the intellectual property rights of content creators. If you are the owner of any material featured on our website and have concerns about its use, please contact us. We are committed to addressing any copyright issues promptly and will remove any material within 2 days of receiving a request from the rightful owner.